|
Introduction
- Diseases are one of the most important factors contributing to low yields in groundnut. Numerous diseases caused by fungi and viruses have been reported in India. Some of them are widely distributed and cause considerable losses while others are restricted to some areas
 Top
Top
Alternaria leaf diseases
Introduction
- TIn India, this disease was first reported from Punjab in 1969. A new disease 'Alternariosis' of groundnut from Tamil Nadu was reported in 1979. The disease was found on plants of all ages but damage was greatest on those nearing maturity. The disease generally occurred in combination with leaf spots and rust.
- Of late, this disease becomes one of the major foliar diseases in some areas like Cuddalore and Villupuram districts. But it is always observed on the post-rainy season irrigated crop.
Fungi
- Alternaria arachidis Kulk., A. alternata (Fr.) Keissler (A. tenuis Auct.) and
A. tenuissima (Kunze ex Pers.) Wilts are the fungi responsible for Alternaria leaf spots.
Epidemiology
- The disease is spread by wind- borne conidia.
Nature and symptoms of damage
 |
- . Lesions produced by Alternaria arachidis are
brown in colour and irregular in shape surrounded
by yellowish halos. Symptoms produced by A. tenuissima
are characterized by blighting of apical portions
of leaflets, which turn light to dark brown in
colour. In the later stages of infection blighted
leaves curl inward and become brittle.
|
- * Lesions produced by A. alternata are small, chlorotic, water soaked and they spread over the surface of the leaf. The lesions become necrotic and brown and are found to be irregular in shape.
- Veins and veinlets adjacent to the lesions become necrotic and the symptom is called veinal necrosis. Lesions increase in area and their central portions become pale, rapidly dry out and disintegrate.
- Affected leaves show chlorosis and in severe attacks become prematurely senescent. Lesions can coalesce, give the leaf a ragged and blighted appearance. Profuse sporulation occurs on the upper surface on mature lesions.
Management
- Spray any one of the following fungicides for control of this disease.
- Mancozeb 1 kg/ha.
- Chlorothalonil 1 kg/ha.
 Top Top
Bud Necrosis Disease (BND)
Introduction
- TIt is also called as peanut spotted wilt, Tomato Spotted Wilt Virus (TSWV), ring mosaic, groundnut mosaic, bunchy top, chlorosis, ring mottle and bud blight. It was first reported in India from Punjab.
- Yield reduction depends on the age of the crop, growth habit, cultivar, soil factors and environmental factors. This disease is common on crops grown in the dry (summer) and rainy seasons in India. It is considered to be an important disease in Coimbatore district of Tamil Nadu.
Casual agent
- Tomato Spotted Wilt Virus (TSWV).
Epidemiology
-
This virus is spread by seven species of thrips. Among them, Frankliniella schultzei is the most efficient vector. Larvae acquire the virus. Adults can not acquire but can transmit the virus. The virus is transmitted in a persistent manner. The virus can be transmitted by mechanical sap inoculations and through grafts. It is not transmitted through groundnut seeds.
- The virus has hosts of more than 200 species in 34 families. In India, tomato, brinjal, blackgram, greengram, Zinnia, Chrysanthemum, Trianthema portulacastrum, Gynandropsis pentaphylla, Ageratum conyzoides, Cassia tora, etc. frequently act as the host plants for this virus.
Nature and symptoms of damage
 |
- Plants infected by necrotic strain are killed
very rapidly. Necrosis on older plants usually
spreads only to the petiole or to the portion
of the stem immediately below the necrotic terminal
bud.
- In the late-infected runner type groundnuts,
a few branches may show mild ring spots or necrosis
of the buds and then the whole plant turns yellow,
wilts and sometimes dies.
- The stunting and proliferation of axillary shoots
are common secondary symptoms. Leaflets formed
on these axillary shoots show reduction in size,
distortion of the lamina, mosaic mottling and
general chlorosis.
|
Very rarely, the lamina is reduced to the midrib, giving
the leaflets a 'shoe-string' appearance. These secondary
symptoms are most common on early-infected plants. |
- This disease is due to a strain of tomato spotted wilt virus. The virus infection is noticed at about 3 weeks after sowing as the young leaves exhibit chlorotic ring spots. As the virus becomes systemic different kinds of malformation of leaves, mosaic patterns and necrosis of terminal buds are seen.
 |
;Seeds from early infected plants are small and shriveled
and their testa show red, brown or purple mottling.
Late infected plants may produce seeds of normal size,
but the testae are often mottled. |
Crop loss
- In early infections it may cause up to 100 per cent loss in yield.
Management
- Clean cultivation is essential. All weeds should be thoroughly eliminated to remove all sources of infection.
- Sorghum as intercrop or barrier crop and cumbu as intercrop with groundnut reduces the disease incidence.
- Adopt closer spacing (15x15 cm) and eliminate infected plants up to 6 weeks after sowing.
- Spray monocrotophos 36 WSC (500 ml /ha) 30 days after sowing either alone or in combination with anti-viral
principles (AVP) from sorghum or coconut leaves. For the preparation of anti-viral principle (AVP), sorghum or
coconut leaves are collected, dried and powdered. To one kg of leaf powder, two litres of water is added and heated
to 60°C for an hour. It is then filtered through muslin cloth and diluted to 10 litres before spraying. Five hundred lit of extract
is required to cover one hectare. Two sprays at 10 and 20 days after sowing are needed.
 Top Top
Collar rot or crown rot and seedling blight
Introduction
- This disease was first reported from Java in 1926. In India, the disease is prevalent in all groundnut tracts and is usually most severe in light sandy soils.
Fungus
- Aspergillus niger Van Tieghem is the causal agent of crown rot.
- The mycelium is hyaline to sub-hyaline. The condiophores arise directly from the substrate and are septate, smooth and thick-walled and hyaline to olive brown in colour. The vesicles are mostly globose and have two rows of hyaline phialides viz., primary and secondary phialides.
- The conidial heads are dark brown to black. The conidia are produced in long chains and are globose, spinulose and dark brown on maturity.
Epidemiology
- The pathogen is carried on the seed surface and in or under the tissues of the testa. The primary sources of inoculum are a) mycelium and spores carried on the seeds. b) plant debris in the soil and c) infected cotyledons or embryos. Seeds become infected during the last days of maturation in the soil and during harvesting, handling and particularly during shelling.
- The disease perpetuates through contaminated soil also. Soil infection spreads to cotyledons or directly to hypocotyl. The seedlings become more susceptible when they are injured.
- The fungus can tolerate low moisture levels and develops well at temperatures between 30° and 35°C. It builds up in soils continuously cropped with groundnut. * Conditions, which are not conducive for quick seed germination such as deep sowing, high soil and air temperatures and chemical toxicity increase the disease incidence. Spreading varieties are more susceptible than bunch varieties.
- The disease is particularly high when the weather during the harvesting period is wet and it increases the seed-borne infection.
Nature and symptoms of damage
 |
- In a moist soil, seeds may be attacked immediately
after sowing leading to pre-emergence rotting.
If the ungerminated seeds are removed from the
soil they are found covered with masses of black
conidia which give the seed a sooty appearance.
Post-emergence infection often culminates in death
and rapid decay of seedlings.
- The first symptom in emerged seedlings is usually
rapid drying of the entire plant. Affected tissues
are covered by sporulating fungus at the soil
surface.
|
- As the infection spreads, the entire collar region become dark brown and shredded. When the infection occurs at hypocotyl region just below the cotyledons, the plant may wilt temporarily but eventually recover.
- Mature plants are also attacked. Lesions develop on the stem just below the soil surface and spread upward along the branches. Beacause of the woodiness of mature plants, symptoms are not generally observed until permanent wilting of branches on the plant is apparent. The dead and dried branches are easily detached from the disintegrated collar region. The fungus sporulates on the surface of mature pods resulting in patches of black sooty spores.
Management
- Harvested pods should be dried promptly.
- Deep planting of seed should be avoided.
- Avoid mechanical damage to the pods and kernels.
- Destruction of infected plant debris.
- Deep ploughing of field.
- Spanish bunch variety J 11 is found resistant to crown rot
- Seed treatment with carbendazim (2 g/ kg) or thiram (4 g/ kg) minimizes the disease incidence.
'
 Top Top
Dry root rot
Introduction
-
It is known by several names such as wilt, charcoal rot, ashy stem blight and root rot. It was first reported in India in 1927. It is prevalent in almost all the groundnut growing localities in India.
Fungus
-
The disease is caused by Macrophomina phaseolina (Tassi) Goid., the sclerotial stage of the fungus is Rhizoctonia bataticola (Taub.) Butler. The fungus produces hyaline to dull brown mycelium. The sclerotia are thick walled and dark brown in colour.
Epidemiology
-
The fungus is primarily seed and soil- borne and persists in soil for longer period as actively growing mycelia or dormant sclerotia. The longevity of pycnidiospores in soil and in infected plant debris is dependent on soil moisture and soil temperature.
- Secondary spread occurs through sclerotia carried through irrigation water, implements and farm operations.
- It prefers high temperature and/or low soil moisture for its activity and infection.
- The pathogen has a wide host range including many leguminous crops such as sesame, sunflower, castor, maize, sorghum, blackgram, greengram, redgram, cowpea, soyabean, cotton, etc.
Nature and symptoms of damage
- The disease may appear at any stage of crop development. In the early stages of infection reddish brown, water soaked lesions appear on the stem just above the ground level. Later the lesions darken and the disease spreads upward to the aerial parts and down into the roots.
- If the lesions girdle the stem, wilting occurs. The dead tissue is covered with abundant sclerotia giving the appearance of a light covering of soot. Pycnidia are also formed in some cases.
- Roots are also affected and they rot. The root tissues are found disintegrated.
- Affected plants come off very easily from the soil and the barks peel off while pulling out. The root bark of affected plant shreds and studded with numerous black sclerotia.
- Pegs and pods are also attacked and are covered with fungal spores leading to 'blacknuts' formation. In the pods the shills as well as kernels are affected. Due to infection in the kernel, the fungus grows between the cotyledons. The fungal growth on kernels becomes black and it produces many minute sclerotia.
- The quality grades of affected kernels are reduced. Such affected kernels will have high fatty acid content, which reduces the value of kernels.
- Dry rot affected pods and kernels are prone for infection by secondary parasites such as Aspergillus flavus.
Management
- Non-host crops may be chosen as inter or mixed crops or be included in the rotation to reduce the soil inoculum and subsequently the disease.
- As and when the diseased and dried plants are noticed in the field they should be removed along with the roots and burnt.
- Treat the seeds with carbendazim 2 g/ kg or
- Trichoderma viride 4 g/kg or
- Pseudomonas fluorescens 10 g / kg.
- Spot drenching with carbendazim @ 0.5 g/lit of water as and when the disease is noticed in the field.
- Soil application of Pseudomonas fluorescens @ 2.5 kg /ha after mixing it with 50 kg of well-decomposed FYM or sand on 30 days after sowing minimizes the disease incidence.
 Top Top
Groundnut rosette
- This is also known as chlorotic rosette. It was reported from Tamil Nadu in 1967.
Causal agent
- Groundnut Rosette Virus (GRV) is the major and causal agent of the disease in groundnut but it is dependent on the other Groundnut Rosette Assistor Virus (GRAV) for transmission by aphids.
Epidemiology
- Aphis craccivora is the important vector. Virus is acquired by the aphids within 30 minutes of feeding. The virus is also transmitted through graft but not through seed.
Nature and symptoms of damage
- Chlorosis and vein banding are observed on young leaflets. Terminal bud necrosis and concentric chlorotic rings observed in BND are absent. Secondary symptoms are characterized by reduction in the size of leaflets showing general chlorosis, vein banding and dark green patches.
- Field grown plants are slightly stunted with extreme proliferation and rosetting of the secondary shoots. Leaflets are small, cupped, crinkled, malformed and chlorotic.
Management
- Destruction of volunteer groundnut plants.
- Spraying of monocrotophos (500 ml/ha) helps to check the spread of disease.
 Top Top
Kalahasthi Malady
- Tylenchorhynchus brevilineatus Williams is the causal agent of Kalahasti malady and the disease is prevalent in Coimbatore, Erode and Dindigul districts.
Stage and symptoms
 |
- Infected plants appear in patches in the field
and are stunted with greener than normal foliage.
- Small brownish-yellow lesions appear on the
pegs and on young developing pods. The margins
of the lesions are slightly elevated because of
the proliferation of host cells around the lesions.
- Peg length is reduced and in advanced stages
of the disease, the entire pod surface becomes
blackened.
- Discolouration is also observed on roots, but
this is less conspicuous than pod discolouration.
|
- Kernels from diseased pods are apparently healthy, although commonly smaller than normal.
- An avoidable yield loss of 10% was estimated due to this nematode.
Management
- Soil incorporation of either carbofuran 3G @ 33 kg/ha or phorate 10G @ 10 kg/ha reduces the disease incidence.
- Soil application of FYM @ 12.5 t/ha or neem cake @ 500-1000 kg/ha reduces the nematodes in the field.
 Top Top
Peanut Stripe
Causal agent
- Peanut Stripe Virus (PStv)
Epidemiology
- The virus is mechanically sap transmissible. The vector, Aphis craccivora transmits the virus in a non-persistent manner. The virus is transmitted through seed. Seed transmission ranges from 2.7 to 28.8 per cent.
- The virus infects clusterbean, cowpea, greengram, sesame and soyabean.
Nature and symptoms of damage
- The characteristic symptoms exhibited by this virus are striping or even banding along the lateral veins of the peanut leaflets.
- Infected plants show oak leaf pattern or mild banding of mid vein of the leaflets. The diseased plants are stunted.
- Symptoms varies with virus isolate and groundnut cultivar. Necrotic and stripe isolates cause severe reduction.
Management
- As the virus is transmitted through seed, certified seeds should be used. The diseased seeds should not be imported from other countries either for research or for commercial purpose. Strict quarantine measures should be followed to avoid the disease.
- Collateral hosts should not be grown in the vicinity of groundnut crop.
- The disease can be reduced by spraying monocrotophos 36 WSC (500 ml /ha), which controls the insect vector.
 Top Top
Rust
- Rust is considered economically important in almost all groundnut growing areas. Loss will be heavy if the crop is also affected by leaf spots.
Fungus
- Puccinia arachidis Speg. is the causal agent of rust disease.
- The fungus produces only uredial and telial satges. The uredial stage is the most predominant and commonly observed stage. Uredia are predominantly hypophyllus, scattered or irregularly grouped, round ellipsoid or oblong, dark cinnamon brown when mature; ruptured epidermis conspicuous; uredospores are pedicellate, unicellular, yellow, oval or round 16 to 22 x 23 to 29 µm and echinulated with 2 or 3 germpores.
- Telia are hypophylus, 0.2 to 0.3 µm in dia, scattered, naked, chestnut brown becoming grey from germination or almost black and the ruptured epidermis prominent. Teliospores are oblong or ovate in shape with rounded to acute and thickened apex, constricted in the middle (2 celled) and dark brown in colour.
- Pycnial and aecial stages have not been recorded.
Epidemiology
- The pathogen is known to perpetuate, spread and produce severe outbreaks by means of wind-borne uredospores. Uredospores also spread as contamination of seeds and pods. Rainsplash and implement also help in dissemination.
- No alternate host is found to be involved in the life cycle. The pathogen may survive from season to season on volunteer groundnut plants and collateral hosts like Arachis marginata, A. nambyquarae and A. prostrata.
- High relative humidity (above 85%), heavy rainfall and low temperature (20°-25°C) favour the disease development and spread.
Nature and symptoms of damage
 |
- The disease attacks all aerial parts of the
plant except flowers and is usually found when
the plants are about 6 weeks old. Small brown
to orange coloured pustules (uredosori) of 0.5
to 1.4 um dia appear on the lower surface of leaves.
The epidermis ruptures and exposes a powdery mass
of reddish brown uredospores.
- Corresponding to the sori, small, necrotic brown
spots appear on the upper surface of leaves. Rust
pustules on the leaflets cause loss of photosynthetic
tissues and it leads to chlorosis and death of
the whole leaflet.
|
 |
- Leaflets may dry up to give the plant a burnt
appearance. Rust pustules may also be seen on
petioles, stem and on shells of developing pods.
- Late in the season, brown teliosori, as dark
pustules appear among the necrotic patches. In
contrast with the rapid defoliation associated
with leaf spots, leaves infected with rust become
necrotic and dry up, but tend to remain attached
to the plant. The severe infection leads to production
of small and shriveled seeds.
|
Crop loss
-
In India, combined attack of rust
and leaf spots lead to 70 per cent loss in pod yield
while the loss due to rust alone was 52 per cent. Losses
when rust occurred at flowering, pegging, pre-pod forming
and pod forming stages were 49, 41, 31 and 18 per cent
respectively.
Management
- Volunteer groundnut plants should be eradicated.
- Weeds should be kept under control, because heavy growth of weeds may encourage disease development.
- Avoid monocropping of groundnut in endemic areas.
- Grow moderately resistant varieties like ALR 1, ALR 2, ALR 3 and VRI 4.
- Spray any one of the following fungicides.
Tridemorph 500 ml/ha;
mancozeb 1 kg /ha;
chlorothalonil 1 kg /ha;
wettable sulphur 2.5 kg /ha.
 Top Top
Stem and pod rot
Introduction
- Stem and pod rots are considered as potential threat to groundnut production in many warm and humid areas under irrigated conditions. This disease is commonly known as stem rot, southern blight, Sclerotium blight, white mould, Sclerotium rot, Sclerotium wilt, root rot and foot rot.
- In India, the disease occurs sporadically and yield losses may amount to 27% or more.
Fungus
- Sclerotium rolfsii Sacc. is the causal agent of this disease.
- The mycelium of the fungus is septate and hyaline with conspicuous branching at acute angles. The young growing mycelial mass is snow white with a silky lusture. * Sclerotia are at first white, becoming light brown to dark brown at maturity with 0.5 to 1.5 mm in dia.
Epidemiology
- The primary source of inoculum is the sclerotia. This can remain viable in the soil for 2 to 3 years. The fungus also survives as mycelium in the soil only for short periods (1-2 months).
- The mycelium can be seed-borne.
- Moderate to high temperature (25° to 35°C) and moist conditions enhance the disease development. Frequent rains or irrigation and dense foliage canopy increase or prolong high soil moisture, which favour stem rot.
- Monocropping, soils with high organic content and fertility, mechanical or biological damage to stems or pods may increase disease severity. Calcium deficiency predisposes stem rot incidence.
- This pathogen has a wide host range of nearly 500 plant species. Redgram, blackgram, greengram, cowpea, ragi and betelvine are few important crop hosts.
Nature and symptoms of damage
 |
- The pathogen attacks all parts of the plant
but stem infection is the most common and serious,
hence it is named as stem rot.
- The first symptom is the sudden wilting of a
branch, which is completely or partially in contact
with the soil. The junction of the branch with
the stem near the soil level is the most favoured
point of attack and a white coating of fungus
mycelium is formed there.
- Leaves of affected branch become chlorotic and
then turn brown as they rapidly dry out. As the
disease advances a white mycelial web spreads
over the soil and the basal canopy of the plant.
|
- The infected areas of the stem become shredded and the fungal mycelium quickly produces abundant mustard seed like, spherical sclerotia on the surface of the affected plant parts or on the soil surface adjacent to the affected plants. The sclerotia are initially white but soon turn dark brown as they mature. Shredded stem tissue is a typical symptom of stem rot.
- The entire plant may by killed or only 2 or 3 branches may get affected. Pod production from such infected plants is poor.
- Pegs colonized by the fungus first show light to dark brown lesions. They later become shredded and pods become detached and are left in the soil at harvest. Affected young pods show light tan coloured lesions. Severely infected pods are completely covered with a white mycelial mat.
- In some cases, the seeds from diseased pods show a characteristic bluish-grey discolouration of the testa, are known as 'blue-damage'. This is caused by the production of oxalic acid by the pathogen.
Management
- Crop sanitation like burning the crop residues and deep ploughing before sowing is important.
- Crop rotation with corn, cotton, garlic and onion is an effective means of control to minimize soil inoculum.
- Seed treatment with carbendazim @ 2 g /kg or Trichoderma viride @ 4 g / kg of seed.
 Top Top
Storage disease
- Aflatoxin contamination of groundnut is a serious problem in most groundnut
- producing countries. Infection of groundnut kernels by the aflatoxin-producing fungi,
- Aspergillus flavus and A. parasiticus and consequent contamination with aflatoxins, may
- occur before harvest, during post-harvest curing and drying or in storage.
- Aflatoxins are toxic and carcinogenic to wide range of animal species and to humans and their presence in groundnut products presents a serious health problem.
Contamination before harvest
- Several factors are known to predispose pods to invasion by A. flavus. Insects damage to shells and seeds during crop growth, field drying and storage, termite attack facilitate invasion of seeds by A. flavus.
- Pod damage by pod-rotting fungi also facilitates the invasion of seeds by A. flavus.
- Delayed harvesting and slow and irregular field drying also result in invasion by the fungus
- Over maturity, drought stress, lowered seed moisture content and decreased vigour in groundnuts are interrelated and moisture related and they can all contribute to increased susceptibility to A. flavus invasion and aflatoxin formation.
After harvest
- Significant fresh invasion by A. flavus and aflatoxin contamination can also takes place during the post harvest drying period. At harvest groundnut seeds contain about
- 40% moisture and are susceptible to fungal invasion until their moisture content dropsbelow 8%.
- Slow and irregular drying favours fungal invasion and mould deterioration of seeds. The percentage of seeds infected by fungi is always lower under rapid drying than under slow drying and the percentage of pods with visible mould damage is also higher under slow drying.
In storage
- Groundnuts are safe from invasion by A. flavus when their moisture content is below 8%, a level below which most properly dried seeds are stored. However, under poor storage, seeds may become accidentally wetted by rain or absorb atmospheric moisture. Such wetting can result in rapid invasion by A. flavus and other mould fungi with consequent aflatoxin contamination. This is true for both decorticated seeds and seeds stored in the shell.
- The most important factor in growth and aflatoxin production by A. flavus is the moisture or relative humidity surrounding the substrate.
Management
- Crop rotation with cotton, tobacco and some cereals such as rice and millets greatly reduce the build up of soil inoculum.
- Excessive weed growth depletes available soil moisture and cause drought stress, so effective weed control reduces the risk of aflatoxin contamination.
- Avoiding mechanical damage during harvesting and subsequent processing.
- Harvesting at proper maturity.
- Drying the produce in the field as rapidly as possible.
- Preventing rewetting during or after drying.
- Removal of damaged or moulded pods.
- Drying to a safe moisture level (8%) before placing in storage.
- Storage at low temperature and humidity.
- Providing dry, well-ventilated godowns with concrete floors should be useful in preventing contamination during storage of groundnuts.
- Any insect infestation in the storage should be controlled. Fumigation may be carried out with fumigants [methyl bromide or mixtures of ethylene di-bromide and methyl bromide (1:3)] effective against insects and fungi.
- Use of tarpaulins or airtight containers is effective in preventing moisture uptake during transportation.
- Use of packaging materials resistant to insect penetration reduce the risk of aflatoxin contamination.
 Top Top
Tikka leaf spots
Introduction
- Early and late leaf spots have been referred to as Cercospora leaf spots, brown leaf spots, peanut cercosporiosis and tikka leaf spots. These leaf spots are more serious among all groundnut diseases.
Fungi
Early leaf spot - Cercospora arachidicola Hori.
(Perfect stage - Mycosphaerella arachidicola W.A. Jenkins)
Late leaf spot - Phaeoisariopsis personata (Berk. & Curt.) V. Arx.
(Perfect stage - Mycosphaerella berkeleyi W.A. Jenkins)
Cercospora arachidicola
- The fungus is intercellular and do not produce haustoria and become intracellular when host cells die. The conidiophores are pale olivaceous or yellowish brown in colour, short, 1 to 2 septate, unbranched and geniculate and arise in clusters. Conidia are hyaline or pale yellow, obclavate, 4 to 12 septate, 38 to 108 µm x 3 to 6 µm in size with rounded to distinctly truncate base and sub-acute tip.
- The perfect stage of the fungus produces perithecia as ascostromata. Asci are cylindrical to clavate and contain 8 ascospores. Ascospores are hyaline, slightly curved and two celled, apical cell larger than the lower cell.
Phaeoisariopsis personata
- The fungus produces internal and intercellular mycelium. The conidiophores are long and continuous, 1 to 2 septate and geniculate, arise in clusters and are olive brown in colour. The conidia are cylindrical to obclavate, short, measure 18 to 60 µm x 6 to 10 µm, hyaline to olive brown, usually straight or slightly curved with 1 to 7 septa, but mostly 3 to 4 septa.
- The fungus in its perfect stage produces perithecia as ascostromata. Asci are cylindrical to ovate, contain 8 ascospores. Ascospores are 2 celled and constricted at septum and hyaline.
Epidemiology
-
The fungus survives for a long period in the infected plant debris as conidia, dormant mycelia and perithecia in soil. The volunteer groundnut plants also harbour the pathogen. The infected crop debris serves as primary inoculum. Secondary spread is by air-borne conidia. Rain splash also helps in the spread of conidia.
- Prolonged high relative humidity for 3 days, low temperature (25° - 30°C) with dew on leaf surface favour infection and disease development. Heavy doses of nitrogenous and phosphatic fertilizers and deficiency of magnesium in soil increase the severity of disease.
Nature and symptoms of damage
-
The disease occurs on all above ground parts of the plant, more severely on the leaves.
Early leaf spot
 |
- The spots occur early in crop season. Circular
to irregular (1-10 mm dia.), dark brown spots
appear on the upper leaf surface with a bright-
yellow, circular halo. On the lower surface, the
lesions are light brown in colour.
|
 |
- On severity
due to formation of fungal fruiting structures the
upper surface of lesions turns black become chlorotic
and necrotic. The lesions coalesce and leaflets are
shed. The leaf spots appear 3-4 weeks after sowing.
Lesions are also produced on petiole, stem and pegs.
|
Late leaf spot
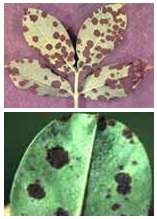 |
- The spots are usually smaller (1-6 mm dia.),
circular and darker in colour than early leaf
spot, without the presence of halo. The lower
surface of the lesions turns to carbon black in
colour.
- The fruiting structures are normally produced
in concentric rings on the lower surface of leaves.
These leaf spots are produced in abundance, when
compared to early leaf spot and spread fast. The
spots appear usually 5-7 weeks after sowing.
|
- Severely infected leaves shed prematurely. Lesions are also produced on petiole, stem and pegs. The quality and yield of pods are drastically reduced in severe infections.
Crop loss
- Yield reduction ranges from 10 to 50 per cent but loss may vary considerably from place to place and between seasons.
Management
-
The plant debris should be removed from the field after harvest or burned in situ or fed to animals. Volunteer groundnut plants should be eradicated.
- Early sowing (especially in kharif) found to record lesser disease incidence.
- Removal of weeds in the fields and in bunds helps to reduce the disease incidence. Grow moderately resistant variety like ALR 1 and ALR 3.
- Spray any one of the following fungicides immediately after the appearance of disease.
- Carbendazim 500 g /ha
- mancozeb 1 kg /ha;
- chlorothalonil 1kg/ha.
- If necessary take up second spray after 15 days.
Management of late leaf spot and rust of groundnut
Foliar spray of Mancozeb (0.1%) + Carbendazim (0.05%) at initiation of disease & 15 days later, reduces LLS and Rust.
Botanicals
- Foliar spray of Calotropis LE10% - two times reduced LLS and Rust.
Biological Control
- One foliar spray of culture filtrate of Penicillium islandicum(50%) at 70 DAS
- Nuclear polyhedrosis virus for the management of Spodoptera and Helicoverpa @ 250 LE/ha
- Spray of aqueous neem leaf extract (2-5%) for the management of leaf spots and rust. Spray of neem seed kernel extract (5%), or crude neem oil (2%) against defoliators, sucking pests and foliar pathogens
- Release of Trichogramma chelonis @ 50000/ha, two times at 7-10 day sinterval followed by release fo Bracon hebetor @ 5000/ha, two times at 7-10 days against leafminer and defoliators.
 Top Top
Yellow mould or Aflaroot disease
Introduction
- Yellow mould is commonly found to affect pods and kernels of groundnut. In the fields, about 5 to 10 per cent of the seedlings showed aflaroot symptoms.
Fungus
- Aspergillus flavus Link ex Fries is the incitant of yellow mould or aflaroot disease. The fungus is both seed and soil-borne.
Nature and symptoms of damage
- Seeds and unemerged seedlings attacked by the fungus are rapidly reduced to a shrivelled, dried, brown or black mass covered by yellow or greenish spores. Decay is most rapid when infected seeds are sown and the fungus becomes active as the seeds hydrate. Seeds disintegrate in 4 to 8 days.
- In the emerging seedling, cotyledons are affected and covered by yellow or greenish-yellow spores leading to death. This phase of the disease is very similar to the crown rot caused by A. niger and in some diseased plants both fungi may be present. *When the strain of A. flavus causing the seedling disease produces aflatoxin, the plants may be severely stunted with chlorotic or pale green leaves with vein clearing of leaflets, that are smaller than usual and have pointed tips. Root development is reduced by infection. This symptom is known as 'aflaroot'.
Crop loss
- In India, a loss of 20 per cent in emergence of seedlings due to seed-borne infection was reported.
Management
- Since the fungus is a weak parasite, agronomic practices, which favour rapid germination and vigorous growth of the seedlings will reduce infection.
 Top Top
Root-Knot Nematode
- Species belonging to 9 genera of plant parasitic nematodes are known to cause injury to groundnut crop.
- Meloidogyne arenaria (Neal) Chitwood, M. hapla Chitwood and M. incognita are the nematodes, which cause root-knots in groundnut crop. These nematodes are prevalent in the districts of Coimbatore, Erode and Dindigul.
Stage and symptomss
- Galls often form on roots, pegs and pods of infected plants.
- Nematode damage is frequently not suspected until roots and pods are examined for the presence of galls, which may reach a diameter of several times the normal adjacent root. Root system development is commonly reduced.
- Severely infected plants are stunted and have chlorotic leaves.
- Pods also become infected and develop knots, or small warts. Pegs and pods occasionally begin to deteriorate at maturity.
Management
- Deep ploughing in summer months up to a depth of 20 cm and fallowing for about one month reduce the population of root-knot nematode.
- Soil application of carbofuran 3G @ 33 kg/ha or phorate 10G @ 10 kg/ha.
- Application of organic amendments like farmyard manure (12.5 t/ha) or neem cake (500-1000 kg/ha) minimises the nematode population in soil.
 Top Top
|
